Determination of Chemical Formula and the Percentage Yield of Product
5b mol O z grams O. Rinse out the crucible with two 8 mL aliquots of distilled water and pour the water into the 50.

How To Calculate Theoretical Yield And Percent Yield Youtube
Percentage Yield Formula Example.
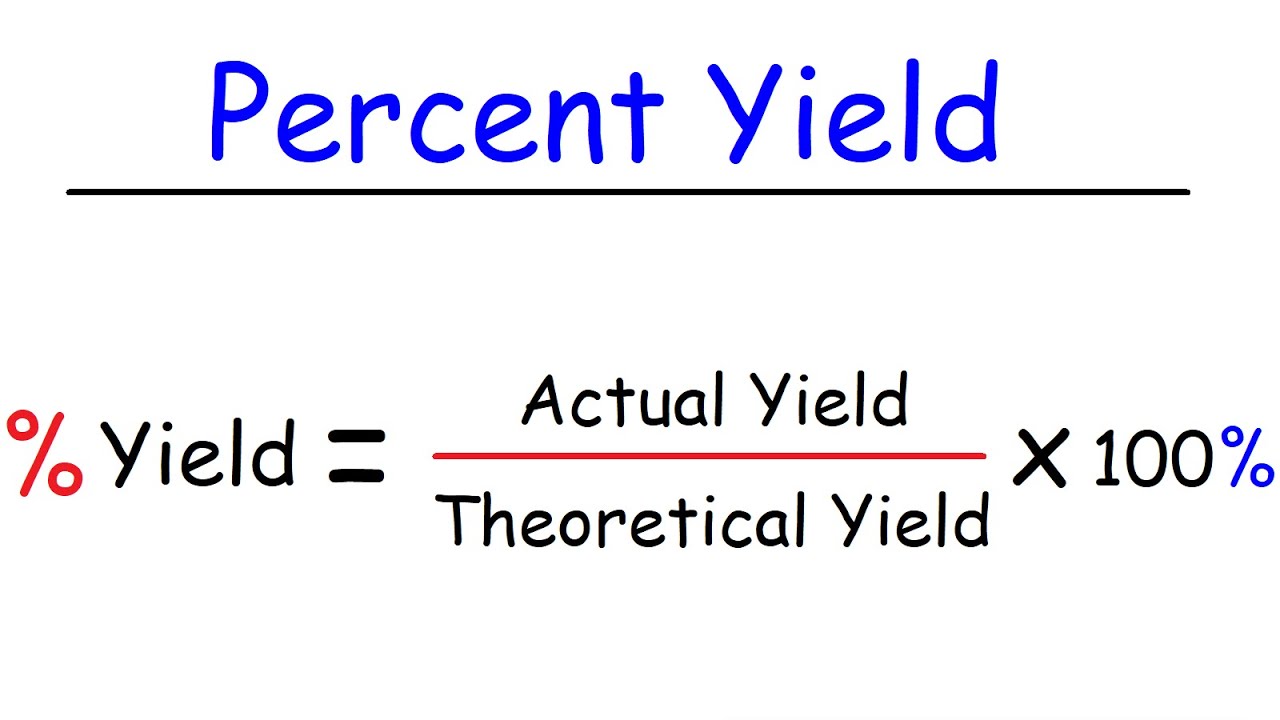
. We know that according to Percent Yield Formula Percentage yield Actual yieldTheoretical yield 100. In experiment-1 the weight of the output product is calculated to be. Identify the actualexperimental yield for the given chemical reaction.
The percent yield is the ration of the actual yield over the theoretical yield times one. In chemistry yield also referred to as reaction yield is a measure of the quantity of moles of a product formed in relation to the reactant consumed obtained in a chemical reaction usually. Plug the yields from Step 1.
345 X4 100. The percent yield of the aspirin obtained from my experiment is 463 yield. The Erlenmeyer flask contains only.
Transfer the brown solid to a clean and empty 50 mL beaker. Where w is the grams of Mg used and z is the grams of O incorporated. Percentage yield 05 x 100.
The formula for percent yield is. The example above shows a three-step method to synthesise Lacosamide an. The mass percentages therefore are expressed as fractions.
The empirical formula of magnesium oxide Mgx Oy is. Percentage yield ActualTheoretical 100. Calculate The Theoretical Yield To Determine The Yield In A Chemical.
Percent yield is the percentage of an actual result to an expected result and reveals the success of the situation in question. RmPercentagermYield fracrmActualrmYieldrmTheoreticalrmYield times 100 Q2. The mass of carbon 2729 g corresponds to 2272 moles of carbon and the mass of oxygen 7271 g corresponds to 4544.
Define Percent Yield by the given Decomposition Reaction. People also downloaded these. Once you have the type of reaction you need a percent yield of a product the percent yield can be found.
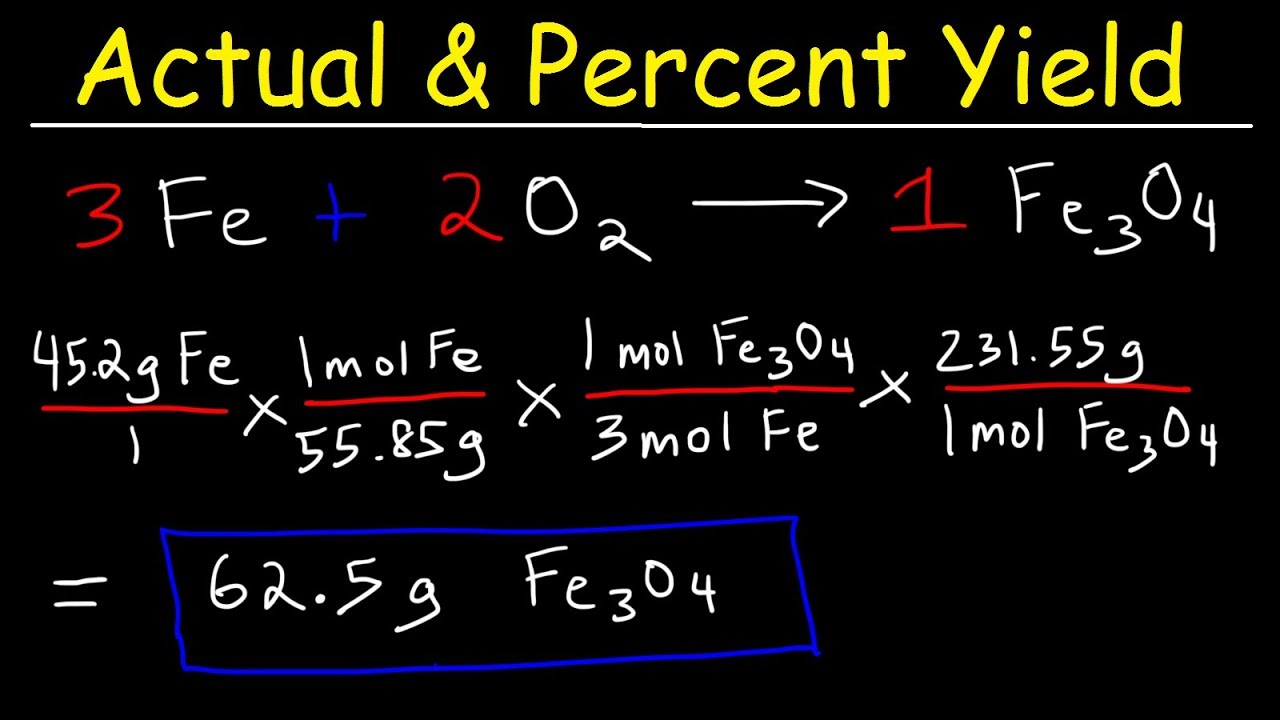
Determine the theoretical yield of the formation of geranyl formate from 465 g of geraniol. View Experiment 11 student versionpdf from CHEMISTRY PRK1026 at University of Malaysia Sarawak. Identify the theoretical yield for the given chemical reaction.
The above reaction shows that for. The product of the zinc-iodine reaction which is soluble in methanol should now be completely transferred from the Erlenmeyer flask to the beaker. Therefore the percentage yield of.
Its formula is. Determination of Chemical Formula and the Percentage Yield of. When you look up cheem research papers the percentage yield of each reaction is written above the arrow.
X 345 4100 138. MgCO 3 MgO CO 2.

How To Calculate Theoretical Yield And Percent Yield Youtube
How To Calculate Theoretical Yield And Percent Yield Youtube

Calculate The Theoretical Yield To Determine The Yield In A Chemical Reaction Youtube
How To Calculate The Percent Yield And Theoretical Yield Youtube
No comments for "Determination of Chemical Formula and the Percentage Yield of Product"
Post a Comment